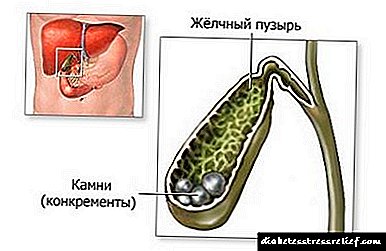
Medicinae Reference
Mutatio de non albo-release tabulas breviuscula ovali notatum, biconvex, laceratum, et sculptum "dia", "LX" huc atque illuc.
| I tab. | |
| gliclazide | LX medicamentum |
Excipients: monohydrate lactose - 71,36 mg forma Maltodextrin - XXII C mg forma hypromellose CP - CLX mg Calcium glycerin - 1.6 mg forma anhydrous colloidal silica - 5.04 emptum.
XV PCs. - papularum, lucentis maculæ (II) - kartonnye.15 pieces sarcinas. - papularum, lucentis maculæ (IV) - cardboard sarcinas.
Pharmacological effectus,
Oralis hypoglycemic coetus II medicamento a generation sulfonylureas quae differunt similis products availability Add N-anulum de quibus heterocyclic endocyclic vinculum.
Diabeton® MB β-cellulis demittit sanguinem GLYCOSA et insulin excremento excitando Langerhans ovarium. Auctus postprandial insulin Mammalia non tenuit, et post II annos C-Lorem. Est effectus in praeter metabolismi carbohydratorum gliclazide hemovascular effectus.
Effectum in secretionem insulin
In type II diabetes (non insulin) praeparatio in montem Domini et mane apicem insulin secretio et augetur secundum tempus in attractio GLYCOSA responsio ad insulin excremento. A significant incremento in insulin excremento occurs in responsione ad excitanda fecit per cibum attractio et GLYCOSA administratione.
Et medicamento potest reducere in periculo thrombosis parva vasa, quæ ad machinationes, ut faciam inpedimenta in diabete: partialis inhibitionis de platelet aggregatio et adhaesio ac decrementum concentration de platelet activum elementum (beta-thromboglobulin, thromboxane B2), et ad reparandum genus fibrinolytic vascularium endothelial actio, et crescat in actione de plasminogen activator tEXTUS.
Intensive glycemic imperium fundatur in usum Diabeton MB (catalytic hemoglobin (HbA1c)